Pd(ii)-catalyzed β- and γ-C-(sp3)–H dienylation with allenyl acetates†
Abstract
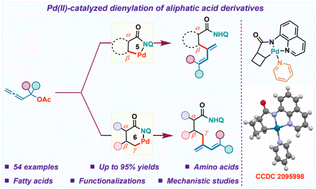
Recent years have seen the emergence of transition metal catalyzed C–H activation as a powerful synthetic tool in organic chemistry. Allenes have fascinated synthetic chemists due to their unique reactivity. While directing group assisted functionalization of C(sp2)–H bonds with allenes is well documented in the literature, their coupling with more challenging aliphatic C(sp3)–H bonds remains elusive. In this regard, we hereby report a Pd(II) catalyzed 8-aminoquinoline directed aliphatic C(sp3)–H dienylation protocol using allenyl acetates. A variety of carboxylic acids including fatty acids and amino acids were efficiently functionalized at β and γ-positions to afford diversely functionalized 1,3-dienes. Preliminary mechanistic studies revealed the crucial role of the base in the success of the transformation. The reaction proceeds via regioselective 2,3-migratory insertion of the allene with the alkylpalladium(II) species followed by β-acetoxy elimination.


 Please wait while we load your content...
Please wait while we load your content...