Development of a continuous synthesis process for carbamazepine using validated in-line Raman spectroscopy and kinetic modelling for disturbance simulation†
Abstract
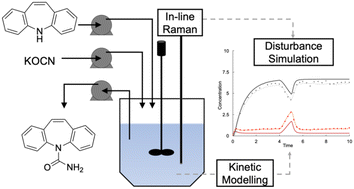
Mitigation of failure modes in the continuous synthesis (CS) of a drug substance (DS) has the potential to widen the adoption of continuous manufacturing (CM) technologies by the pharmaceutical industry. This work demonstrates the development of a robust continuous process for the synthesis of carbamazepine (CBZ), an essential medicine as per the World Health Organization (WHO), facilitated by kinetic modelling and monitored by in-line Raman spectroscopy. Accurate kinetic modelling and the use of validated process analytical technology (PAT) models for quantitative measurement were found to play an important role in developing CS of drug substances. Kinetic data for the formation of CBZ from iminostilbene (ISB) were collected by batch reaction sampling and high-performance liquid chromatography (HPLC) analysis. A non-linear solver and iterative method was applied to determine two sets of Arrhenius parameters simultaneously for the reaction system by minimizing the standard error of the model fit. The start-up and dynamic equilibrium stages for the CS of CBZ using a continuous stirred tank reactor (CSTR) were modelled based on the batch kinetic data and employed to optimize conversion and simulate process disturbances. An in-line Raman spectroscopy method was successfully developed, validated, and integrated to determine the concentrations of CBZ and ISB within the operating range for the CS. The CS kinetic model was evaluated experimentally from startup to dynamic equilibrium over 10 residence times with monitoring by HPLC and in-line Raman spectroscopy. The developed kinetic model in tandem with in-line Raman spectroscopy successfully predicted disturbances due to changes in process variables and can serve as a useful tool in the future design of advanced process control strategies for the continuous synthesis of CBZ.


 Please wait while we load your content...
Please wait while we load your content...