Selective aerobic oxidation of aliphatic aldehydes: the critical role of percarboxylate anion on the selectivity†
Abstract
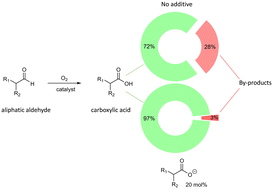
During the aerobic oxidation of aldehydes, peracids accumulate in the reaction mixture. From a safety and selectivity perspective, the quantification and transformation of the peracids into the corresponding carboxylic acids are of vital importance in both laboratory and industrial applications. The presence of peracids in the crude reaction mixture is directly correlated with the selectivity of the oxidation, especially for β-substituted aliphatic aldehydes. We show that 20 mol% of carboxylate salts, added at the beginning of the reaction or generated in situ, catalyzed the selective transformation of the peracids into carboxylic acids and strongly increased the selectivity towards carboxylic acid without decreasing the chemical rate. This methodology increased the safety of the process by preventing the build-up of peracids in the course of the reaction. Otherwise, for aliphatic aldehydes, peracids are non-selectively decomposed during the course of the experiment or workup. We proposed that the presence of carboxylate salt in the reaction mixture improves aerobic oxidation's selectivity by forming a percarboxylate anion. The latter leads to the selective conversion of peracid into the corresponding carboxylic acid.


 Please wait while we load your content...
Please wait while we load your content...