Enriching calixarene functionality with 1,3-diketone groups†
Abstract
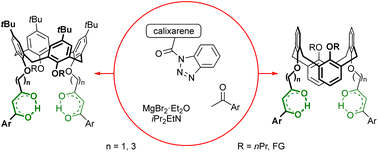
Acylation of magnesium enolates of acetophenones with calixarene-based 1-acylbenzotriazoles was systematically studied to access scarcely available cone and 1,3-alternate calix[4]arenes having the synthetically/receptor valuable 1,3-diketone groups linked to the phenol oxygen atoms of the macrocycles. 1-Acylbenzotriazoles were prepared from the readily available calixarenes bearing acetic or butyric acid residues, which allowed us to link the desired dicarbonyl units to the molecular platform using methylene or propylene groups. The expandability of the synthetic approach towards multifunctional macrocycles was assessed using several series of cone and 1,3-alternate calix[4]arene-based 1-acylbenzotriazoles having crown ether loops or triazole units as additional functionalities. Although all the targeted 1,3-diketone derivatives were obtained, elimination of additional functional residues and partially reversible inter/intramolecular reactions involving pairs of the dicarbonyl groups in the products during their preparation/purification were observed in several cases. For the prepared 1,3-diketones, the tautomeric equilibrium was substantially shifted toward the canonical ketoenol form in solutions, and only such forms were observed in the solid state by X-ray diffraction. Still, uncommon ketoenol tautomers were detected in solutions of cone methylene-linked 1,3-diketones, in which the enolized 1,3-diketone units were not self-conjugated having an OH group hydrogen bonded to the phenol oxygen atoms of the macrocycle. This was proved by X-ray-diffraction for a specially prepared 1-indanone-derived calixarene-based 1,3-diketone, in which the unconjugated ketoenol was the major form due to structural reasons. Within the preliminary complexation study, the ability of deprotonated calixarene bis(1,3-diketones) to bind transition metal cations was confirmed by NMR data for zinc and lanthanide(III) complexes and by X-ray data for a copper(II) complex.


 Please wait while we load your content...
Please wait while we load your content...