Visible-light-initiated nickel-catalyzed amination of aryl halides using thioxanthen-9-one as a photocatalyst†
Abstract
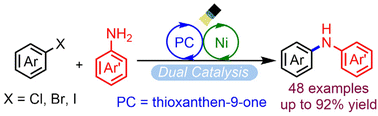
This paper describes a mild strategy for amination of aryl halides with anilines by dual photoredox and nickel catalysis. Inexpensive and commercially available thioxanthen-9-one significantly accelerated this cross-coupling, providing a diverse range of diarylamines in good yields (41–93%) at room temperature. This reaction proceeded under compact fluorescent lamp light or sunlight and was tolerant of a wide range of functional groups. It was applied to the synthesis of natural product and drug molecules, and proceeded efficiently on a gram-scale. The mechanism involves oxidative addition of an aryl halide to nickel(0), transmetalation of aryl-Ni(II) halide, energy transfer from triplet photosensitizer thioxanthen-9-one to an aryl-Ni(II)-amino intermediate, and subsequent reductive elimination to diarylamine.


 Please wait while we load your content...
Please wait while we load your content...