Double C–H bond functionalization for C–C coupling at the β-position of thiophenes using palladium-catalyzed 1,4-migration associated with direct arylation†
Abstract
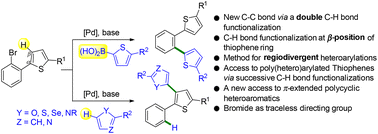
The functionalization of C–H bonds at β-positions of 5-membered ring heteroarenes such as thiophene is generally more challenging than at α-positions. By using Pd-catalyzed 1,4-migration associated with direct arylation, under appropriate conditions, the functionalization of such thienyl β-positions of 2-arylthiophenes is possible. The oxidative addition of 2-(2-bromoaryl)thiophenes to palladium followed by Pd 1,4-migration activates these β-positions. Then, Pd-catalyzed direct coupling with heteroarenes provides β-heteroarylated 2-arylthiophene derivatives. In the course of this coupling reaction, a new C–C bond arises from the functionalization of two C–H bonds. Conversely, the Suzuki reaction using such 2-(2-bromoaryl)thiophenes provides 1,2-diheteroaryl-substituted benzene derivatives. These regiodivergent heteroarylations tolerate a range of substituents on the benzene ring and also several heteroarenes and provide a new route to π-extended polycyclic heteroaromatics. Moreover, these procedures employ easily available air-stable catalysts and inexpensive bases.


 Please wait while we load your content...
Please wait while we load your content...