Stereoselective semisynthesis of uzarigenin and allo-uzarigenin†
Abstract
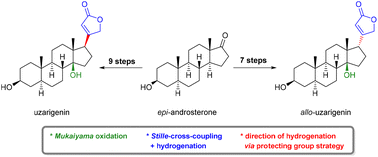
Herein, we report the concise semisynthesis of the natural cardenolide uzarigenin and its diastereoisomer allo-uzarigenin in nine and seven steps, respectively, starting from the broadly available epi-androsterone. For this purpose, the synthetic strategy for the stereoselective introduction of the β-hydroxy group at C-14 via Mukaiyama oxidation is discussed. Additionally, the installation of the butenolide ring at C-17 is performed using a Stille-cross-coupling reaction with subsequent stereoselective hydrogenation of the C-16/C-17 double bond to exclusively give allo-uzarigenin. By directing the hydrogenation via a protecting group strategy, the C-17β isomer can also be obtained stereoselectively.
- This article is part of the themed collection: Recent Open Access Articles in Frontiers Journals


 Please wait while we load your content...
Please wait while we load your content...