Base-mediated chalcogenoaminative annulation of 2-alkynylanilines for direct access to 3-sulfenyl/selenyl-1H-indoles†
Abstract
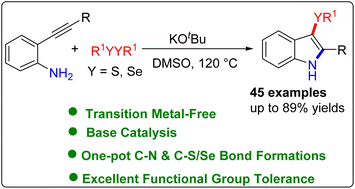
An efficient and transition metal-free synthesis of 3-sulfenyl/selenyl-1H-indoles via a base-assisted chalcogenoaminative annulation of 2-alkynyl aniline with disulfides/diselenides is described. A series of 2-alkynylanilines were found compatible with dichalcogenides in this transformation providing 3-sulfenyl/selenyl-1H-indoles in good to excellent yields. The presented methodology has the advantages of easily available raw materials, functional group tolerance, and a wide range of substrates that provide access to 3-sulfenylindoles and 3-selenylindoles.


 Please wait while we load your content...
Please wait while we load your content...