Abstract
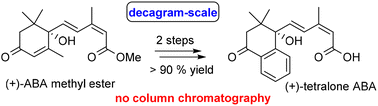
The plant hormone (S)-abscisic acid (ABA) is a signalling molecule found in all plants that triggers plants’ responses to environmental stressors such as heat, drought, and salinity. Metabolism-resistant ABA analogs that confer longer lasting effects require multi-step syntheses and high costs that prevent their application in crop protection. To solve this issue, we have developed a two-step, efficient and scalable synthesis of (+)-tetralone ABA from (S)-ABA methyl ester. A challenging three-carbon insertion and a bicyclic ring formation on (S)-ABA methyl ester was achieved through a highly regioselective Knoevenagel condensation, cyclization, and oxidation in one-pot. Further we have studied the biological activity and metabolism of (+)-tetralone ABA in planta and found the analog is hydroxylated similarly to ABA. The biologically active hydroxylated tetralone ABA has greater persistence than 8′-hydroxy ABA as cyclization to the equivalent of phaseic acid is prevented by the aromatic ring. (+)-tetralone ABA complemented the growth retardation of an Arabidopsis ABA-deficient mutant more effectively than (+)-ABA. Taken together, this new synthesis allows the production of the potent ABA agonist efficiently on an industrial scale.


 Please wait while we load your content...
Please wait while we load your content...