Friedel–Crafts arylation of aldehydes with indoles utilizing arylazo sulfones as the photoacid generator†
Abstract
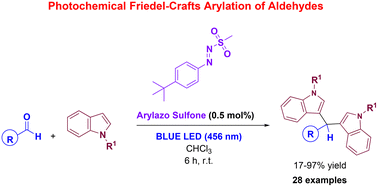
A versatile, inexpensive and sustainable protocol for the preparation of valuable bis-indolyl methanes via visible light-mediated, metal-free Friedel–Crafts arylation has been developed. The procedure, that exploits the peculiar behavior of arylazo sulfones as non-ionic photoacid generators (PAGs), was applied to the conversion of a variety of aliphatic and aromatic aldehydes into diarylmethanes in good to highly satisfactory yields, employing a low-catalyst loading (0.5 mol%) and irradiation at 456 nm.


 Please wait while we load your content...
Please wait while we load your content...