Electrochemically enhanced deoxygenative cross-coupling of aryl ketones with heteroarenes through in situ generated benzyl carbocations†
Abstract
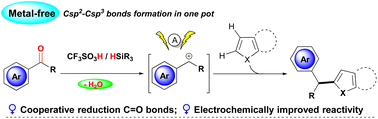
Triflic acids/silanes as cooperative reductants enable the convenient transformation of C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O bonds through a multistep reaction pathway in one pot. Electrolysis of the acidic reaction mixture significantly improved carbonyl reduction and thus facilitated the generation of benzyl carbocations, which show high reactivity towards electron-rich heteroarenes for C–C bond formation.
O bonds through a multistep reaction pathway in one pot. Electrolysis of the acidic reaction mixture significantly improved carbonyl reduction and thus facilitated the generation of benzyl carbocations, which show high reactivity towards electron-rich heteroarenes for C–C bond formation.


 Please wait while we load your content...
Please wait while we load your content...