Palladium-catalyzed C(sp3)–H nitrooxylation of masked alcohols†
Abstract
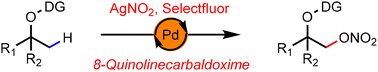
A palladium-catalyzed β-C(sp3)–H nitrooxylation of aliphatic alcohols with AgNO2 is reported. An 8-formylquinoline-derived oxime is installed as an exo-type directing group for sp3 C–H activation and selectfluor acts as the oxidant. The reaction tolerates a variety of functional groups and shows good selectivity for β-C–H nitrooxylation of alcohols.


 Please wait while we load your content...
Please wait while we load your content...