Novel irreversible peptidic inhibitors of transglutaminase 2†
Abstract
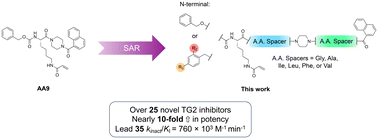
Transglutaminase 2 (TG2), also referred to as tissue transglutaminase, plays crucial roles in both protein crosslinking and cell signalling. It is capable of both catalysing transamidation and acting as a G-protein, these activities being conformation-dependent, mutually exclusive, and tightly regulated. The dysregulation of both activities has been implicated in numerous pathologies. TG2 is expressed ubiquitously in humans and is localized both intracellularly and extracellularly. Targeted TG2 therapies have been developed but have faced numerous hurdles including decreased efficacy in vivo. Our latest efforts in inhibitor optimization involve the modification of a previous lead compound's scaffold by insertion of various amino acid residues into the peptidomimetic backbone, and derivatization of the N-terminus with substituted phenylacetic acids, resulting in 28 novel irreversible inhibitors. These inhibitors were evaluated for their ability to inhibit TG2 in vitro and their pharmacokinetic properties, and the most promising candidate 35 (kinact/KI = 760 × 103 M−1 min−1) was tested in a cancer stem cell model. Although these inhibitors display exceptional potency versus TG2, with kinact/KI ratios nearly ten-fold higher than their parent compound, their pharmacokinetic properties and cellular activity limit their therapeutic potential. However, they do serve as a scaffold for the development of potent research tools.


 Please wait while we load your content...
Please wait while we load your content...