Expanding the structure–activity relationships of alkynyl diphenylurea scaffold as promising antibacterial agents†
Abstract
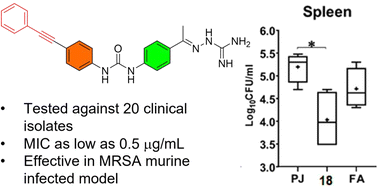
With the continuous and alarming threat of exhausting the current antimicrobial arsenals, efforts are urgently needed to develop new effective ones. In this study, the antibacterial efficacy of a set of structurally related acetylenic-diphenylurea derivatives carrying the aminoguanidine moiety was tested against a panel of multidrug-resistant Gram-positive clinical isolates. Compound 18 was identified with a superior bacteriological profile than the lead compound I. Compound 18 demonstrated an excellent antibacterial profile in vitro: low MIC values, extended post-antibiotic effect, refractory ability to resistance development upon extended repeated exposure, and high tolerability towards mammalian cells. Finally, when assessed in a MRSA skin infection animal model, compound 18 showed considerable healing and less inflammation, decrease in the bacterial loads in skin lesions, and it surpassed fusidic acid in controlling the systemic dissemination of S. aureus. Collectively, compound 18 represents a promising lead anti-MRSA agent that merits further investigation for the development of new anti-staphylococcal therapeutics.
- This article is part of the themed collection: Antimicrobial Resistance


 Please wait while we load your content...
Please wait while we load your content...