β-(Z)-Selective alkyne hydrosilylation by a N,O-functionalized NHC-based rhodium(i) catalyst†
Abstract
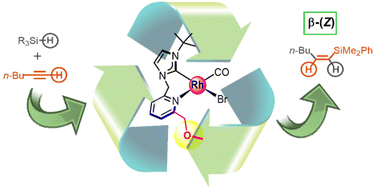
Neutral and cationic cyclooctadiene rhodium(I) complexes with a lutidine-derived polydentate ligand having NHC and methoxy side-donor functions, [RhBr(cod)(κC-tBuImCH2PyCH2OMe)] and [Rh(cod)(κ2C,N-tBuImCH2PyCH2OMe)]PF6, have been prepared. Carbonylation of the cationic compound yields the dicarbonyl complex [Rh(CO)2(κ2C,N-tBuImCH2PyCH2OMe)]PF6 whereas carbonylation of the neutral compound affords a mixture of di- and monocarbonyl neutral complexes [RhBr(CO)2(κC-tBuImCH2PyCH2OMe)] and [RhBr(CO)(κ2C,N-tBuImCH2PyCH2OMe)]. These complexes efficiently catalyze the hydrosilylation of 1-hexyne with HSiMe2Ph with a marked selectivity towards the β-(Z)-vinylsilane product. Catalyst [RhBr(CO)(κ2C,N-tBuImCH2PyCH2OMe)] showed a superior catalytic performance, in terms of both activity and selectivity, and has been applied to the hydrosilylation of a range of 1-alkynes and phenylacetylene derivatives with diverse hydrosilanes, including HSiMe2Ph, HSiMePh2, HSiPh3 and HSiEt3, showing excellent β-(Z) selectivity for the hydrosilylation of linear aliphatic 1-alkynes. Hydrosilylation of internal alkynes, such as diphenylacetylene and 1-phenyl-1-propyne, selectively affords the syn-addition vinylsilane products. The β-(Z) selectivity of these catalysts contrasts with that of related rhodium(I) catalysts based on 2-picolyl-functionalised NHC ligands, which were reported to be β-(E) selective. An energy barrier ΔG‡ of 19.8 ± 2.0 kcal mol−1 (298 K) has been determined from kinetic studies on the hydrosilylation of 1-hexyne with HSiMe2Ph. DFT studies suggest that the methoxy-methyl group is unlikely to be involved in the activation of hydrosilane, and then hydrosilane activation is likely to proceed via a classical Si–H oxidative addition.


 Please wait while we load your content...
Please wait while we load your content...