Models of the putative antimony(v)–diolate motifs in antileishmanial pentavalent antimonial drugs†
Abstract
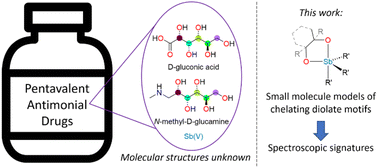
The structures of the pentavalent antimonials, small-molecule Sb-containing drugs used to treat the neglected tropical disease leishmaniasis, remain unknown despite their widespread use for over half a century. These drugs are prepared by combination of an Sb(V) precursor and a sugar derivative and proposed structures frequently invoke a cyclic stiborane motif in which a vicinal diolate ligand chelates an Sb(V) center. As a step towards better understanding the structures of the pentavalent antimonial drugs, a series of cyclic organostiboranes spanning the stereochemical space afforded by a vicinal diolate motif has been synthesized and characterized. X-ray crystallography and NMR spectroscopy provide unambiguous characterization of the structures of these model compounds and of the interaction of the diolate with the Sb(V) center. Particularly notable are the systematic trends observed in the NMR spectroscopic signals as a function of the stereochemistry of the diolate. The spectroscopic signatures identified with these model compounds will provide a framework for elucidating the structures of the pentavalent antimonial drugs.


 Please wait while we load your content...
Please wait while we load your content...