Mechanistic insights into the effect of halide anions on electroreduction pathways of CO2 to C1 product at Cu/H2O electrochemical interfaces†
Abstract
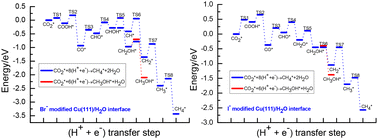
Various elementary reaction steps during CO2 electroreduction into C1 product are systematically studied at specifically adsorbed halide anions modified Cu(111)/H2O interfaces via theoretical calculations with the aim of identifying the effect of halide anions X− (X = F, Cl, Br, I) on CO2 electroreduction reaction activity, mechanisms and product selectivity in this work. Our present studies show that halogen atoms can be adsorbed on the Cu electrodes during CO2 electroreduction, thus leading to notable electronic interactions between halogen atoms and Cu(111)/H2O interface. It is found that halogen atoms can gain electrons in the order of F > Cl > Br > I, showing that the adsorbed halide anions can be formed. The presence of halide anions can notably be favor of CO formations. CO electroreduction pathways towards C1 product at Br− and I− modified Cu(111)/H2O interfaces are examined due to poor selectivity of CO electroreduction into CHO at F− and Cl− modified Cu(111)/H2O interfaces. The calculated results indicate that the presence of Br− and I− facilitate CO2 electroreduction into C1 product since notably enhanced CO2 electroreduction activity can be achieved, which may be ascribed to the formations of chemically adsorbed anion radical ˙CO2− and more positive onset potentials for CO formations. Notably, it is found that the electroreduction pathways of CO2 into CH4 and CH3OH product may be able to parallelly occur at Br− and I− modified Cu(111)/H2O interfaces, whereas only CH4 production pathways can occur at clean Cu(111)/H2O interface. Thus, it can be concluded that the presence of halogen anions on Cu alter mechanism and product selectivity of CO2 electroreduction. Our present mechanistic insights into this effect may be able to give a theoretical guideline for control of mechanisms and product selectivity during CO2 electroreduction.


 Please wait while we load your content...
Please wait while we load your content...