A central functional group-dependent stereoinduction mechanism for chiral super Brønsted C–H acid catalysis†
Abstract
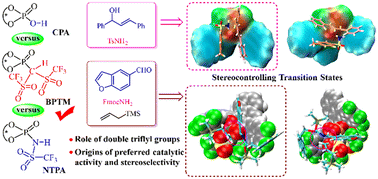
The recently reported chiral BINOL-derived phosphoryl bis-((trifluoromethyl)sulfonyl)methane (BPTM) is a promising super Brønsted acid for efficient C–C and C–X coupling (X = heteroatom), placing a compelling need for deep understanding of the underlying mechanism and a model of effective stereoinduction to guide the design and optimization of novel catalytic systems. Herein, an in-depth computational investigation is conducted to unravel the mechanisms of BPTM promoted asymmetric allylic amination, which is then compared to that catalyzed by another two chiral BINOL-derived Brønsted acids, N-triflyl phosphoramides (NTPA) and phosphoric acids (CPA). A central functional group (CFG)-controlled asymmetric stereoinduction mechanism is proposed to rationalize the effect of catalyst structural variation on the enantioselectivity, which revealed the role of the CFG [P(![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C(Tf)2)OH] of BPTM in three aspects: (1) creates beneficial hydrogen-bonding interactions (C–H⋯O, N–H⋯O, and C–H⋯F) in the chiral cavity essential for inducing high enantioselectivities; (2) regulates the orientation of the substrates to induce a more favorable chiral environment than in the cases of NTPA and CPA; (3) improves the electrophilicity of the reaction sites of the substrates and facilitates the initial water elimination step. The distortion–interaction analysis further confirms the governing role of the CFG. The CFG stereoinduction model is applied to explain the origin of enantioselectivity of a three-component coupling reaction, and the more favorable noncovalent interactions (C–H⋯F and C–H⋯π) and chiral environment contributed by the CFG are identified as the most vital beneficial factors to the enantioselectivity. The present study provides insights into the correlation of the CFG of BPTM with high stereoinduction and is expected to inspire future novel design of effective chiral Brønsted catalysts.
C(Tf)2)OH] of BPTM in three aspects: (1) creates beneficial hydrogen-bonding interactions (C–H⋯O, N–H⋯O, and C–H⋯F) in the chiral cavity essential for inducing high enantioselectivities; (2) regulates the orientation of the substrates to induce a more favorable chiral environment than in the cases of NTPA and CPA; (3) improves the electrophilicity of the reaction sites of the substrates and facilitates the initial water elimination step. The distortion–interaction analysis further confirms the governing role of the CFG. The CFG stereoinduction model is applied to explain the origin of enantioselectivity of a three-component coupling reaction, and the more favorable noncovalent interactions (C–H⋯F and C–H⋯π) and chiral environment contributed by the CFG are identified as the most vital beneficial factors to the enantioselectivity. The present study provides insights into the correlation of the CFG of BPTM with high stereoinduction and is expected to inspire future novel design of effective chiral Brønsted catalysts.


 Please wait while we load your content...
Please wait while we load your content...