Enhancing the performance for palladium catalysed tert-butyl hydroperoxide-mediated Wacker-type oxidation of alkenes†
Abstract
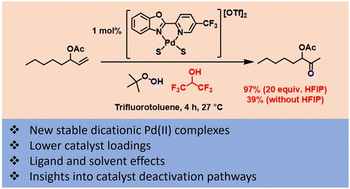
This work examines the palladium(II) catalysed oxidation of terminal alkenes to their corresponding methyl ketones using tert-butyl hydroperoxide (TBHP) as the oxidant. The study aimed to reduce catalyst loadings and to understand some of the factors which are important in the design of more effective methods. A series of ligands based around 2-(2-pyridyl)benzoxazole (PBO) were studied and a new dicationic catalyst was developed which can operate more efficiently than previously reported catalysts. The choice of solvent system was also found to have a significant impact on catalyst performance. In the case of oct-1-en-3-yl acetate, a model substrate for a challenging class of substrates (protected allylic alcohols), it was found that using 1,1,1,3,3,3-hexafluoroisopropanol (HFIP), as part of a solvent mixture, greatly improved the reaction; enabling shorter reaction times and lower catalyst loadings.


 Please wait while we load your content...
Please wait while we load your content...