Chlorine-radical-mediated C–H oxygenation reaction under light irradiation
Abstract
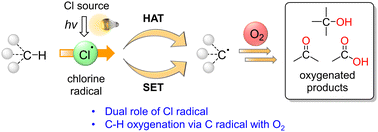
The oxygenation of C(sp3)–H bonds is a key chemical reaction in the production of fine chemicals and pharmaceuticals. Hydrogen atom transfer has recently gained significant attention for its ability to functionalise C–H bonds. The C–H bond can be activated by transferring the H radical to a hydrogen acceptor such as the chlorine radical (Cl˙). Thus, the Cl˙ generated by light irradiation can be used to initiate C–H oxygenation reactions, in which molecular oxygen is used as the oxidant. In this review article, we have summarised the recent advances in the field of Cl˙-mediated C–H oxygenation reactions. Reactions with catalysts such as metal chlorides, organophotoredox catalysts (acridinium ions), and chlorine dioxide radicals under light irradiation have been discussed. We conclude the review by providing suggestions for future research studies in the field. We expect that this review article will provide valuable information for the development of Cl˙-mediated C–H oxygenation reactions, contribute to the understanding of the reactivity of Cl˙, and inspire other useful synthetic chemical applications for C–H oxygenation reactions.


 Please wait while we load your content...
Please wait while we load your content...