Tuning the aqueous self-assembly of porphyrins by varying the number of cationic side chains†
Abstract
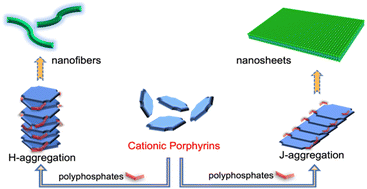
Due to their excellent electronic and optical properties, porphyrins are extensively studied conjugated macrocycles in supramolecular chemistry for assembling functional nanomaterials. Although the aggregation of monomers plays a significant role in driving the self-assembly process into ordered nanostructures, it remains a challenge for tuning the self-assembling behavior of porphyrins through molecular structure modifications, especially in aqueous solutions. In the present work, two novel water-soluble porphyrin derivatives were synthesized by introducing cationic linear side chains into the π-conjugated core for phosphate-templated assembly through electrostatic interactions. It was found that the stacking patterns (H- or J-type aggregation) of porphyrins could be tuned by varying the number of side chains, which are associated with dramatic morphological change. The cytotoxicity and photodynamic properties of the J-aggregation-driven nano-assemblies were also investigated for the purpose of anti-cancer treatment. This study demonstrates a facile and effective strategy to regulate the aqueous self-assembling behavior of porphyrins that can impact the structure and properties of assembly, which will be of great benefit to the design and synthesis of functional nanomaterials for specific applications.

 Please wait while we load your content...
Please wait while we load your content...