Origin of the unique thermoelectric transport in Mg3(Sb,Bi)2: absence of d-orbital bonding in crystal cohesion†
Abstract
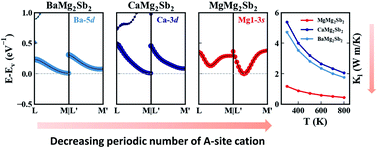
The Zintl phase compound Mg3(Sb,Bi)2 has attracted much research interest, owing to its excellent thermoelectric performance comparable to n-type Bi2Te3-based materials. Among the family of AMg2X2 (A = Mg, Ca, Sr, Ba, Yb; X = Sb, Bi), Mg3(Sb,Bi)2 exhibits very exceptional thermoelectric behavior. It is the only member being experimentally n-type doped, and its lattice thermal conductivity is significantly lower than those of the other members, such as CaMg2Sb2 and YbMg2Bi2. Band structure calculations revealed that the conduction band minimum of AMg2X2 is located at the high symmetry ‘M’ point, whereas Mg3(Sb,Bi)2 is an exception. Here, we present a systematic theoretical study to clarify the origin of the anomalous behavior of Mg3(Sb,Bi)2. It is revealed that the thermoelectric transport of the other AMg2X2 compounds is dominated by the strong p–d bonding between the ‘X’ site and ‘A’ site. In Mg3(Sb,Bi)2, however, the d orbital bonding is absent due to the small period number of Mg. The weak ‘A–X’ site bonding in Mg3(Sb,Bi)2 is proven to be responsible for all the unusual properties. Based on the new understanding, BaMg2SbBi is proposed as a new candidate for n-type compounds for its relatively weak p–d bonding. Our work resolves the puzzling behaviors of Mg3(Sb,Bi)2 and provides an insight into the thermoelectric transport of the whole AMg2X2 family.


 Please wait while we load your content...
Please wait while we load your content...