The impact of carbonation on hydroxide diffusion in nano-confined anion exchange membranes†
Abstract
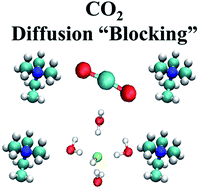
Exposing anion exchange membrane (AEM) fuel cells to ambient air is known to decrease fuel cell efficiency significantly due to the presence of CO2. In this combined theoretical and experimental study, we examine the hydration conditions that promote reactions between CO2 and hydroxide ions in nano-confined AEMs, and we explore the effect of the carbonation process on the solvation structure and diffusion of hydroxide ions. Using fully atomistic ab initio molecular dynamics (AIMD) simulations, we find that increasing hydration can delay the carbonation reaction between OH− and CO2. Once reacted, HCO3− ions exist along the simulation, which significantly reduce the diffusion of the hydroxide ions. We confirm these results using 1H- and 13C-pulsed field gradient nuclear magnetic resonance. AIMD simulations further reveal that the HCO3− actively “blocks” the diffusion path of hydroxide ions along the simulation cell. We expect that elucidating the key design principles underlying the atomistic effects of carbonate ions on hydroxide ions diffusion mechanisms in model AEMs will provide useful guidelines for the synthesis and experimental characterization of novel highly conductive AEM fuel cell technologies in the presence of CO2-containing air.


 Please wait while we load your content...
Please wait while we load your content...