Designing a descriptor for the computational screening of argyrodite-based solid-state superionic conductors: uniformity of ion-cage size†
Abstract
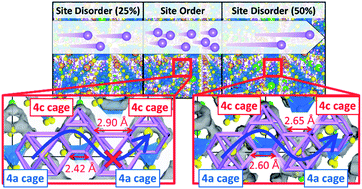
The use of solid electrolytes is a promising way to improve the energy density of lithium-ion batteries, and Li argyrodites make up a promising family of solid-state electrolytes with comparable Li-ion conductivities to liquid electrolytes. However, the vast number of compositions potentially available from the periodic table (Li7+y−x[(AyB1−y)S4]S2−xXx (0 ≤ x ≤ 1), where A, B and X are elements of group IV, V, and VI, respectively) poses an overwhelming challenge for researchers to find the best chemical combinations and structures. Therefore, it is essential to design a simple descriptor for the computational screening of Li argyrodite-based superionic conductors. Recently, it was suggested that altering the halogen distribution in Li argyrodites during synthesis could increase the Li-ion conductivity of these materials due to site disorder of S2−/X− single anions. Inspired by this work, we systematically investigated the “composition–structure–property” relationship in Li6−xPS5−xX1+x (0 ≤ x ≤ 1 and X = Cl, Br or I) model structures. Our results show a close correlation between the Li-ion conductivity and the cage-like Li sublattice structure around the S2−/X− single anions. We particularly found that the size of the Li-ion cage becomes uniform with increasing the halogen doping level, and the inter-cage diffusion of Li ions is accelerated to increase Li-ion conductivity. Therefore, we propose a standard deviation (STD) of Li-cage size around S2−/X− single anions as a descriptor for the screening of argyrodite-based superionic conductors. Furthermore, this work provides a correction method for accurate bulk ionic conductivity calculations considering all possible site disorder configurations and crystallinities of Li argyrodite materials. Our results will provide a novel approach for tuning the compositional change of Li argyrodites based on “composition–structure–property” relationships that accelerate inter-cage diffusion to increase Li-ion conductivity.


 Please wait while we load your content...
Please wait while we load your content...