Glycerol oxidation-assisted electrochemical CO2 reduction for the dual production of formate†
Abstract
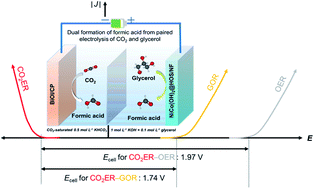
The sluggish oxygen evolution reaction (OER) is one of the main bottlenecks for efficient CO2 electroreduction (CO2ER). Seeking a suitable organic oxidation reaction with a lower redox potential to replace the OER is a promising method to boost the overall efficiency of the CO2ER. Here, we propose a method of substituting the OER with a glycerol oxidation reaction (GOR) for the CO2ER to achieve the coproduction of formate at both the cathode and anode. A two-electrode GOR-assisted CO2ER system is successfully established with a Ni foam-supported surface-sulfurized nickel–cobalt hydroxide nanoneedle catalytic anode (Ni0.33Co0.67(OH)2@HOS/NF) and a BiOI cathode. The simultaneous formation of formate from the anodic GOR and cathodic CO2ER with faradaic efficiencies (FEs) of 90% and 92%, respectively, is obtained at a cell voltage of 1.9 V (22.4 mA cm−2). More significantly, an overall electricity-to-formate energy conversion efficiency of 110% is obtained in our GOR-assisted CO2ER system. This work not only proposes an energy- and atom-efficient method for the CO2ER but also provides new insights for developing highly active non-noble metal catalysts for the GOR.


 Please wait while we load your content...
Please wait while we load your content...