An electrochemical multicomponent reaction toward C–H tetrazolation of alkyl arenes and vicinal azidotetrazolation of alkenes†
Abstract
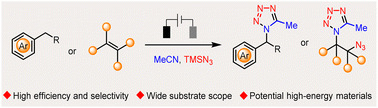
The widespread use of tetrazoles in medicine, biology, and materials science continuously promotes the development of their efficient and selective syntheses. Despite the prosperous development of multicomponent reactions, the use of the most abundant and inexpensive chemical feedstocks, i.e., alkanes and alkenes, toward the preparation of diverse tetrazoles remains elusive. Herein, we developed an electrochemical multicomponent reaction (e-MCR) for highly efficient and selective C–H tetrazolation of alkyl arenes. When applied to alkenes, the corresponding vicinal azidotetrazoles were readily obtained, which were further demonstrated to be versatile building blocks and potential high-energy materials.


 Please wait while we load your content...
Please wait while we load your content...