Multiple remote C(sp3)–H functionalizations of aliphatic ketones via bimetallic Cu–Pd catalyzed successive dehydrogenation†
Abstract
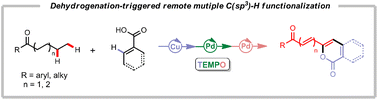
The dehydrogenation-triggered multiple C(sp3)–H functionalizations at remote positions γ, δ or ε, ζ to carbonyl groups of aliphatic ketones with aryl/alkenyl carboxylic acids as coupling partners have been achieved using a bimetallic Cu–Pd catalyst system. This reaction allows access to alkenylated isocoumarins and their derivatives in generally good yields with high functional group tolerance. The identification of bimetallic Cu–Pd synergistic catalysis for efficient successive dehydrogenation of aliphatic ketones, which overcomes the long-standing challenge posed by the successive dehydrogenation desaturation of terminally unsubstituted alkyl chains in aliphatic ketones, is essential to achieving this bimetallic Cu–Pd catalyzed dehydrogenation coupling reaction.


 Please wait while we load your content...
Please wait while we load your content...