Radical 1,2,3-tricarbofunctionalization of α-vinyl-β-ketoesters enabled by a carbon shift from an all-carbon quaternary center†
Abstract
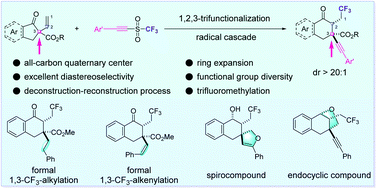
Herein, we report an intermolecular, radical 1,2,3-tricarbofunctionalization of α-vinyl-β-ketoesters to achieve the goal of building molecular complexity via the one-pot multifunctionalization of alkenes. This reaction allows the expansion of the carbon ring by a carbon shift from an all-carbon quaternary center, and enables further C–C bond formation on the tertiary carbon intermediate with the aim of reconstructing a new all-carbon quaternary center. The good functional group compatibility ensures diverse synthetic transformations of this method. Experimental and theoretical studies reveal that the excellent diastereoselectivity should be attributed to the hydrogen bonding between the substrates and solvent.
- This article is part of the themed collection: 2022 Chemical Science HOT Article Collection


 Please wait while we load your content...
Please wait while we load your content...