Construction of an α-chiral pyrrolidine library with a rapid and scalable continuous flow protocol†
Abstract
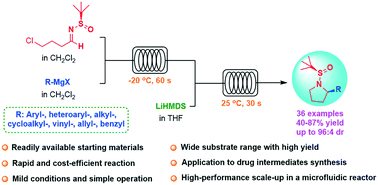
An α-chiral pyrrolidine library has been established via a highly diastereoselective continuous flow protocol under mild conditions. Various functionalized pyrrolidines have been obtained in generally high yields (up to 87%) and with superior diastereocontrol within 150 s. The utility of this flow methodology is further demonstrated by the gram-scale preparation of a κ-opioid receptor selective antagonist Aticaprant intermediate. Moreover, the scale-up in a self-designed microfluidic reactor provides a throughput of 7.45 g h−1, suggesting its potential large-scale applications. The flow procedure affords rapid, cost-efficient, and scalable access to obtain chiral α-substituted pyrrolidines in the compound library.


 Please wait while we load your content...
Please wait while we load your content...