Analysis of n-hexane, 1-hexene, cyclohexane and cyclohexene catalytic cracking over HZSM-5 zeolites: effects of molecular structure†
Abstract
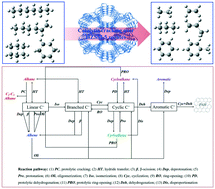
In order to reveal the effects of molecular structure, the catalytic cracking of n-hexane, 1-hexene, cyclohexane and cyclohexene over HZSM-5 zeolites was carried out at 260–550 °C under an atmosphere. Particular attention was paid to the variation in the trends of the conversion and product distribution with the reaction temperature and time on stream (TOS). The fresh and spent HZSM-5 zeolites were studied by XRD, SEM, Py-IR, TPO, NH3-TPD and N2 physisorption. It was found that the catalytic activity was in descending order of 1-hexene > cyclohexene > n-hexane > cyclohexane, and the catalytic stability was in descending order of 1-hexene > n-hexane > cyclohexene > cyclohexane. n-Hexane benefited alkane formation, 1-hexene benefited alkene formation, while cyclohexane and cyclohexene benefited aromatic formation. Coke formation blocked the porous channels and reduced the acid sites of HZSM-5 zeolites, and C6 catalytic cracking exhibited a distinct response to increases in coke and TOS. Compared with n-hexane, the increase in coke formation significantly inhibited cyclohexane catalytic cracking. Although 1-hexene catalytic cracking achieved a similar amount of coke to cyclohexane, the conversion of 1-hexene remained unchanged with an increase in TOS, which was attributed to the high activity of the C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C bond. Analogously, cyclohexene with high activity exhibited stable conversion with an increase in TOS. Interestingly, the coke was almost all generated at the beginning in cyclohexene catalytic cracking at 550 °C and selectivity for everything except benzene was close to zero after 3 h on stream. Considering the stable conversion and fluctuating product distribution with an increase in TOS, it was deduced that alkene was crucial to coke formation in cyclohexene catalytic cracking. The typical reaction pathways were summarized from the literature and mechanism indices were defined to reveal the role of molecular structure in C6 catalytic cracking. It was found that the C
C bond. Analogously, cyclohexene with high activity exhibited stable conversion with an increase in TOS. Interestingly, the coke was almost all generated at the beginning in cyclohexene catalytic cracking at 550 °C and selectivity for everything except benzene was close to zero after 3 h on stream. Considering the stable conversion and fluctuating product distribution with an increase in TOS, it was deduced that alkene was crucial to coke formation in cyclohexene catalytic cracking. The typical reaction pathways were summarized from the literature and mechanism indices were defined to reveal the role of molecular structure in C6 catalytic cracking. It was found that the C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C bond and cyclic structure inhibited protolytic cracking, hydride transfer and isomerization, while they enhanced oligomerization and aromatization compared to a C–C bond and a linear structure, respectively.
C bond and cyclic structure inhibited protolytic cracking, hydride transfer and isomerization, while they enhanced oligomerization and aromatization compared to a C–C bond and a linear structure, respectively.


 Please wait while we load your content...
Please wait while we load your content...