Visible light-induced photoredox catalyzed C–N coupling of amides with alcohols†
Abstract
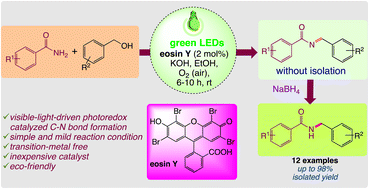
A visible-light-mediated method for the construction of N-monoalkylated products from easily available benzamides and benzyl alcohol in the presence of eosin Y has been developed. The reaction proceeded smoothly, for a wide range of derivatives of benzamides and benzyl alcohols, to give the desired products in good to excellent yields. Biological studies, such as those on drug-likeness and molecular docking, are carried out on the molecules.


 Please wait while we load your content...
Please wait while we load your content...