DTBP-mediated cross-dehydrogenative coupling of 3-aryl benzofuran-2(3H)-ones with toluenes/phenols for all-carbon quaternary centers†
Abstract
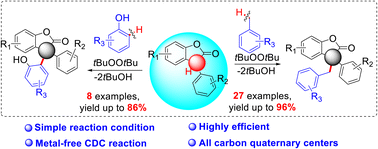
We have developed a transition-metal free protocol for efficient cross-dehydrogenative coupling of 3-aryl benzofuran-2(3H)-ones and toluenes/phenols using DTBP as an oxidant. A diverse range of 3-aryl benzofuran-2(3H)-ones, toluenes, and phenols undergo C–H bond cleavage to generate all-carbon quaternary centers in good yields, making this protocol useful for the synthesis of complex molecules. A gram scale experiment was performed in good yield.


 Please wait while we load your content...
Please wait while we load your content...