Iron salt-promoted oxidation of steroidal phenols by m-chloroperbenzoic acid: a route to possible antitumor agents†
Abstract
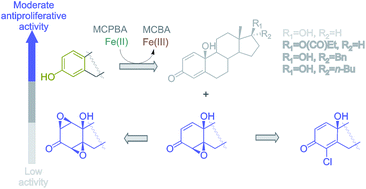
A new oxidant, containing m-chloroperoxybenzoic acid (MCPBA) and an iron salt, was developed and used for oxidation of steroidal phenols to a quinol/epoxyquinol mixture. Reaction was optimized for estrone, by varying initiators (Fe-salts), reaction temperature, time and mode of MCPBA application. A series of five more substrates (17β-estradiol and its hydrophobized derivatives) was subjected to the optimized oxidation, providing corresponding p-quinols and 4β,5β-epoxyquinols in good to moderate yields. The obtained epoxyquinols were additionally transformed by oxidation, as well as the acid-catalyzed oxirane opening. In a preliminary study of the antiproliferative activity against human cancer cell lines, all newly synthesized compounds expressed moderate to high activity.


 Please wait while we load your content...
Please wait while we load your content...