Quality by design approach for green HPLC method development for simultaneous analysis of two thalassemia drugs in biological fluid with pharmacokinetic study†
Abstract
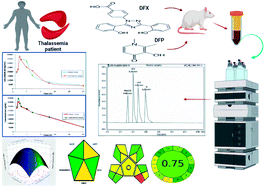
This work implements a combined experimental approach of analytical quality-by-design (AQbD) and green analytical chemistry (GAC) to develop an HPLC method for simultaneous determination of the two thalassemia drugs, deferasirox (DFX) and deferiprone (DFP), in biological fluid for the first time. This integration was designed to maximize efficiency and minimize environmental impacts, as well as energy and solvent consumption. To accomplish this goal, an analytical quality-by-design approach was performed, beginning with quality risk assessment and scouting analysis, followed by Placket–Burman design screening for five chromatographic parameters. Critical method parameters were thoroughly recognized and then optimized by using a two levels-three factors custom experimental design to evaluate the optimum conditions that achieved the highest resolution with acceptable peak symmetry within the shortest run time. The desirability function was used to define the optimal chromatographic conditions, and the optimal separation was achieved using an XBridge® HPLC RP-C18 (4.6 × 250 mm, 5 μm) column with ethanol : acidic water at pH 3.0 adjusted by phosphoric acid in the ratio of (70 : 30, v/v) as the mobile phase at a flow rate of 1 mL min−1 with UV detection at 225 nm at a temperature of 25 °C. Linearity was obtained over the concentration range of 0.30–20.00 μg mL−1 and 0.20–20.00 μg mL−1 for DFX and DFP, respectively, using 20.00 μg mL−1 ibuprofen (IBF) as an internal standard. The established method's greenness profile was evaluated and measured using various assessment tools, and the developed method was green. For the validation of the developed method, FDA recommendations were followed, and all the results obtained met the acceptance criteria. The suggested method was successfully used to study the pharmacokinetic parameters of DFX and DFP in rat plasma. Due to the substantial increase in bioavailability of the two iron chelating drugs, the results from this study strongly recommend their co-administration.


 Please wait while we load your content...
Please wait while we load your content...