Cu(ii)-thiophene-2,5-bis(amino-alcohol) mediated asymmetric Aldol reaction and Domino Knoevenagel Michael cyclization: a new highly efficient Lewis acid catalyst†
Abstract
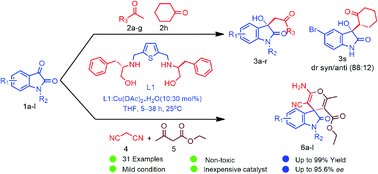
The highly efficient Lewis acid-catalytic system Cu(II)-thiophene-2,5-bis(amino-alcohol) has been developed for enantioselective Aldol reaction of isatin derivatives with ketones. The new catalytic system also proved to be highly enantioselective for the one pot three-component Domino Knoevenagel Michael cyclization reaction of substituted isatin with malononitrile and ethylacetoacetate. The chiral ligand (2S,2′S)-2,2′-((thiophene-2,5-diylbis(methylene))bis(azanediyl))bis(3-phenylpropan-1-ol) (L1) in combination with Cu(OAc)2·H2O employed as a new Lewis acid catalyst, furnished 3-substituted-3-hydroxyindolin-2-ones derivatives (3a–s) in good to excellent yields (81–99%) with high enantioselectivities (up to 96% ee) and spiro[4H-pyran-3,3-oxindole] derivatives (6a–l) in excellent yields (89–99%) with high ee (up to 95%). These aldol products and spiro-oxindoles constitute a core structural motif in a large number of pharmaceutically active molecules and natural products.


 Please wait while we load your content...
Please wait while we load your content...