Iodine-DMSO mediated conversion of N-arylcyanothioformamides to N-arylcyanoformamides and the unexpected formation of 2-cyanobenzothiazoles†‡
Abstract
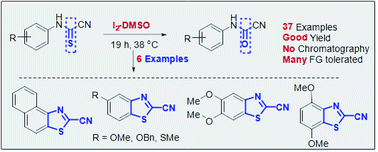
Cyanoformamides are ubiquitous as useful components for assembling key intermediates and bioactive molecules. The development of an efficient and simple approach to this motif is a challenge. Herein, we demonstrate the effectiveness of the I2-DMSO oxidative system in the preparation of N-arylcyanoformamides from N-arylcyanothioformamides. The synthetic method features mild conditions, broad substrate scope, and high reaction efficiency. Furthermore, this method provides an excellent entry to exclusively afford 2-cyanobenzothiazoles which are useful substrates to access new luciferin analogs. The structures of all new products were elucidated by multinuclear NMR spectroscopy and high accuracy mass spectral analysis. Crystal-structure determination by means of single-crystal X-ray diffraction was carried out on (4-bromophenyl)carbamoyl cyanide, 5,6-dimethoxybenzo[d]thiazole-2-carbonitrile, 5-(benzyloxy)benzo[d]oxazole-2-carbonitrile, 4,7-dimethoxybenzo[d]thiazole-2-carbonitrile, and (5-iodo-2,4-dimethoxyphenyl)carbamoyl cyanide, a key intermediate with mechanistic implications.


 Please wait while we load your content...
Please wait while we load your content...