Synthesis of dihydroisoquinolinone-4-methylboronic esters via domino Heck/borylation using a structurally characterized palladacycle as a catalyst †
Abstract
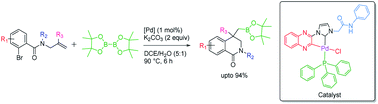
Synthesis of dihydroisoquinolinone-4-methylboronic esters from N-allylcarboxamides and B2(Pin)2 via domino Heck/borylation approach is reported. A quinoxaline-based NHC-palladacycle [Pd(C∧C:)PPh3Cl], which has been structurally characterized, is used as a catalyst. The scope of the substrate with a wide range of substituents is explored. In addition to the synthesis of title compounds, a few examples of methylboronic esters of indoline and benzofuran motifs have also been prepared using the same protocol.


 Please wait while we load your content...
Please wait while we load your content...