Regioselective oxidative C–H heptafluoroisopropylation of heteroarenes with heptafluoroisopropyl silver†
Abstract
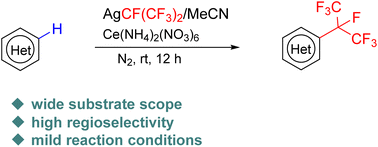
While CF(CF3)2-containing arenes are prevalent in pharmaceuticals and agrichemicals, CF(CF3)2-containing heteroarenes remain largely underexplored. Herein, we disclose the first oxidative C–H heptafluoroisopropylation of heteroarenes using AgCF(CF3)2 as the CF(CF3)2 source and ceric ammonium nitrate (CAN) as the oxidant. This protocol provides a practical and efficient approach towards a diverse array of CF(CF3)2-substituted heteroarenes with excellent regioselectivity. The synthetic utility of this protocol is well documented in the late-stage heptafluoroisopropylation of pharmaceutical molecules.


 Please wait while we load your content...
Please wait while we load your content...