Harvesting the fragment-based nature of bifunctional organocatalysts to enhance their activity†
Abstract
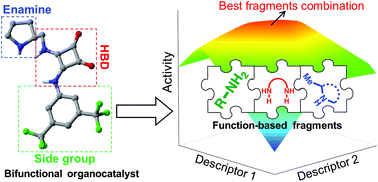
Bifunctional hydrogen-bond donors/amines are commonly encountered organocatalysts in asymmetric synthesis. Existing computational tools do not take full advantage of their modularity to suggest tailored designs because they generally rely on evaluating the organocatalyst's structure as a whole. Herein, we introduce a fragment-based approach, coupled with volcano plots and activity maps, to evaluate hundreds of building block combinations and extract mechanistic insight. This bottom-up protocol gives feedback on the choice of improved molecular fragments and makes activity-based screening faster and more transferable.


 Please wait while we load your content...
Please wait while we load your content...