Direct access to 2-aryl-3-cyanothiophenes by a base-catalyzed one-pot two-step three-component reaction of chalcones with benzoylacetonitriles and elemental sulfur†
Abstract
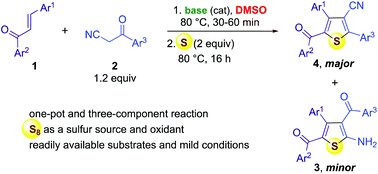
3-Cyanothiophene is an important heterocyclic scaffold in bioorganic and medicinal chemistry as a useful synthetic intermediate as well as in materials science as a privileged motif for photovoltaic development. Herein, we report our unexpected results on the formation of 3-cyanothiophene derivatives as the major products via a three-component reaction of chalcones, benzoylacetonitriles and elemental sulfur along with the minor products 2-aminothiophenes. The ratios between these two thiophene products is 4 : 3 and could be varied by simply changing the promoting base as well as its stoichiometric ratio. The method was successfully extended to benzoylacetate in place of benzoylacetonitrile to provide thiophene-3-carboxylates.


 Please wait while we load your content...
Please wait while we load your content...