Direct and efficient synthesis of tetrasubstituted allenyl organothiophosphates from propargylic alcohols under catalyst- and additive-free conditions†
Abstract
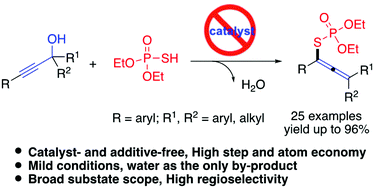
An environment-friendly approach that affords tetrasubstituted allenyl organothiophosphates containing highly congested carbon centers from easily prepared propargylic alcohols and phosphorothioic acids [(RO)2P(O)SH] with water as the only by-product is developed. The reaction is carried out by an in situ dehydrative cross-coupling process involving the allenyl carbocation intermediate and followed by nucleophilic attack to achieve high product distribution. In addition, the reaction occurred in the presence of (EtO)2P(O)SH, and no ligand, additive or additional acid promoter was needed. It is noted that (EtO)2P(O)SH acts not only as an acid promoter but also as a nucleophile. Moreover, a variety of propargylic alcohols bearing electron-rich and electron-withdrawing groups were well tolerated to generate various tetrasubstituted allenyl organothiophosphates in moderate to excellent yields under mild conditions.


 Please wait while we load your content...
Please wait while we load your content...