Visible-light-mediated defluorinative cyclization of α-fluoro-β-enamino esters catalyzed by 4-CzIPN†
Abstract
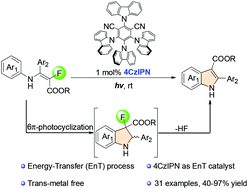
Using 4-CzIPN as an energy transfer (EnT) photocatalyst and α-fluoro-β-enamino esters as substrates, a mild 6π-photocyclization/defluorination of N-aryl enamines was carried out to efficiently construct indoles without an oxidant and a transition metal catalyst under visible light irradiation. Mechanistic studies reveal that the triplet N-aryl enamines produced through energy transfer from the excited 4-CzIPN* are responsible for the formation of indoles.


 Please wait while we load your content...
Please wait while we load your content...