Visible light-mediated radical-cascade addition/cyclization of arylacrylamides with aldehydes to form quaternary oxindoles at room temperature†
Abstract
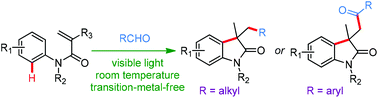
A visible-light-mediated oxidative cyclization of N-arylacrylamides by a radical cascade with readily available aliphatic or aromatic aldehydes was achieved. This transformation provides an operationally convenient, mild, and efficient access to various alkyl-substituted or acyl-substituted oxindoles at room temperature under metal-free conditions. This protocol features good functional group tolerance and high chemoselectivity with general yields ranging from moderate to good. Mechanistic studies reveal that the bromo radical probably plays a promotional role in the formation of the acyl radical via HAT.


 Please wait while we load your content...
Please wait while we load your content...