Tandem-catalysis-enabled highly chemoselective deoxygenative alkynylation and alkylation of tertiary amides: a versatile entry to functionalized α-substituted amines†
Abstract
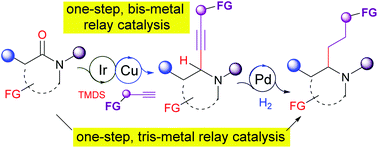
We report here the highly chemoselective catalytic reductive alkynylation and reductive alkylation of tertiary amides to give propargylamines and α-branched amines, respectively. The method features a tandem iridium (Vaska's complex)-catalyzed hydrosilation of amides and copper(I)-salt-catalyzed addition of terminal alkynes. The combination of this relay catalysis with Pd/C-catalyzed hydrogenation constitutes a one-step formal catalytic reductive alkylation of amides. By virtue of this relay catalysis strategy, the in situ catalytic generation and subsequent addition of alkynyl carbanions was achieved. Additionally, the reductive alkylation highlighted the use of feedstock alkynes as surrogates of highly reactive, non-stabilized alkyl carbanions. The method is high yielding and shows remarkable chemoselectivity and functional group tolerance on both the amide and alkyne partners. The value and versatility of this methodology was demonstrated via the one-step synthesis of an intermediate in the synthesis of berkeleyamide A and the one-step racemic synthesis of piperidine alkaloid isosolenopsin.


 Please wait while we load your content...
Please wait while we load your content...