Copper-mediated three-component synthesis of 2-trifluoromethyl benzimidazoles†
Abstract
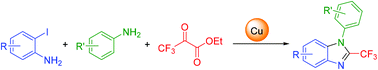
A copper-mediated three-component reaction of o-iodoanilines, anilines, and ethyl trifluoropyruvate is reported. The transformation allows diverse substrate scope on both o-iodoanilines and anilines, delivering various 2-trifluoromethylbenzimidazole products in moderate to good yields. Mechanistic studies suggest that this transformation proceeds via condensation of o-iodoaniline with ethyl trifluoropyruvate, followed by an intermolecular Ullmann-type cross-coupling reaction with aniline and intramolecular amination.


 Please wait while we load your content...
Please wait while we load your content...