Efficient and eco-friendly oxidative cleavage of C–C bonds of 1,2-diols to ketones: electrochemistry vs thermochemistry†
Abstract
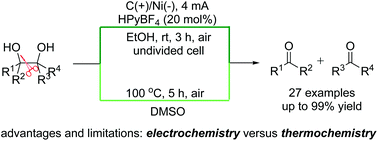
Two efficient methods for the oxidative cleavage of C–C single bonds of vicinal tertiary diols by electrochemical and thermochemical strategies have been independently developed. The corresponding ketone products are smoothly assembled under transition-metal catalyst free and exogenous-oxidant free conditions with up to 99% isolated yield. The advantages and limitations of organic electrosynthesis and thermochemical synthesis for this specific reaction are discussed and compared. The current studies demonstrate that these two reaction systems pass through different reaction mechanisms, and the former is much greener and more efficient.


 Please wait while we load your content...
Please wait while we load your content...