Theoretical investigation of the α-substitution effect on γ-C(sp3)–H arylation of amines: structure–reactivity relationship (SRR) studies†
Abstract
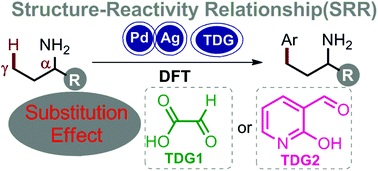
Structure–reactivity relationship (SRR) studies to understand the α-substitution effect toward Pd-catalyzed γ-C(sp3)–H arylation enabled by catalytic transient directing groups have carefully been performed by DFT calculations. Selected transient directing groups of glyoxylic acid (TDG1) and 2-hydroxy-nicotinaldehyde (TDG2) were used in this study. With the increase in the number of substituents on the α-position of amines, the calculated free energy barriers of the rate-determining transition states decreased, in accordance with the experimental results showing that the reactions were largely limited to substrates with α-quaternary centers and reduced yields were observed with amines bearing no α-substituent. This could be attributed to the Thorpe–Ingold effect, where the introduction of substituents on the α-position naturally brings the γ-C(sp3)–H bond close to the Pd center, thus facilitating the C–H bond activation process. Distortion/interaction analysis was also carried out to elucidate the origin of the γ-C(sp3)–H bond reaction barriers. The calculated results indicate that the reaction barriers can be mainly attributed to the distortion of the fragment with the amine substrate. This systematic study paves the way to effectively design catalytic systems toward the remote γ-C(sp3)–H functionalization of primary amines.


 Please wait while we load your content...
Please wait while we load your content...