Enantioselective construction of a congested quaternary stereogenic center in isoindolinones bearing three aryl groups via an organocatalytic formal Betti reaction†
Abstract
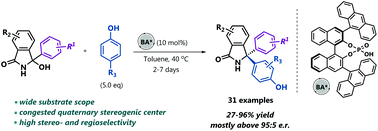
An efficient enantioselective formal Betti reaction between phenols and diaryl ketimines generated in situ from isoindolinone alcohols is described. In a reaction catalyzed by a chiral phosphoric acid, a broad range of ketimines and phenols afforded isoindolinone derivatives comprising a congested quaternary stereogenic center bearing three aryl groups in high yields, and high regioselectivities and enantioselectivities.


 Please wait while we load your content...
Please wait while we load your content...