Catalyst-free aziridine-based step-growth polymerization: a facile approach to optically active poly(sulfonamide amine)s and poly(sulfonamide dithiocarbamate)s†
Abstract
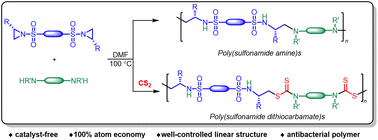
A highly facile catalyst-free synthetic methodology for optically active polysulfonamide derivatives with controlled linear architecture has been established based on chiral bis(2-substituted N-sulfonyl aziridine)s. Novel poly(sulfonamide amine)s are prepared in good yields (65–99%) by the step-growth polymerization of bis(aziridine) and diamine. A one-pot, two-step protocol for the synthesis of poly(sulfonamide dithiocarbamate)s has been developed by using the in situ generated bis(dialkyldithiocarbamate), from diamine and carbon disulfide, as the nucleophile to react with bis(aziridine). With appropriate solvents used in this approach, the branches or cross-linkages derived from the homo-polymerization of aziridines are avoided. The synthesized polysulfonamides exhibit excellent thermal properties with decomposition temperature at 5% weight loss (Td,5%) up to 314 °C, and glass transition temperature (Tg) ranging from 49.9 to 137.5 °C. The poly(sulfonamide amine)s bearing tertiary amino groups demonstrate almost complete killing of Staphylococcus aureus (Gram-positive) and Escherichia coli (Gram-negative). It is anticipated that this aziridine-based step-growth polymerization approach will promote aziridine-related polymer chemistry and benefit the exploration of potential applications of aziridine-derived polymeric materials.


 Please wait while we load your content...
Please wait while we load your content...