Investigating miscibility and lithium ion transport in blends of poly(ethylene oxide) with a polyanion containing precisely-spaced delocalized charges†
Abstract
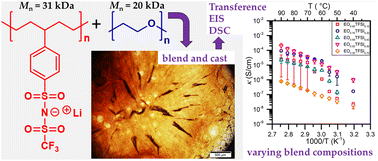
A novel precision single-ion conductor with phenylsulfonyl(trifluoromethylsulfonyl)imide lithium salt covalently bound to every fifth carbon of a polyethylene backbone, p5PhTFSI-Li, was synthesized via ring opening metathesis polymerization (ROMP) followed by post polymerization modification. The conversion of poly(4-phenylcyclopentene), bearing 94% sulfonate anions, to trifluoromethanesulfonimide (TFSI) anions was highly efficient (∼90%) as determined by 19F NMR analysis and corroborated through other spectroscopic methods. The flexible hydrocarbon backbone combined with a bulky TFSI anion led to an observable glass transition temperature of 199 °C even at these high levels of ionization. A high thermal stability up to 375 °C was also observed. Blending of p5PhTFSI-Li with poly(ethylene oxide) at various compositions was performed to investigate electrochemical performance and transference numbers with respect to the lithium electrode using a combination of impedance and polarization methods. At 90 °C and a 50 : 50 wt% blend composition, this system displayed the highest reported conductivity (2.00 × 10−4 S cm−1) of a system with a demonstrated lithium-ion transference number near unity. Such performance is also atypical of single ion conductors produced through post-polymerization modification, which we attribute to the high yield of TFSI conversion. Investigations into the complex miscibility and phase behavior of these blends at various compositions was also probed by a combination of microscopy and differential scanning calorimetry, which is discussed with reference to computational predictions of how charge correlations affect polymer blend phase behavior.


 Please wait while we load your content...
Please wait while we load your content...